To find carbon, look in the ocean
If you need lab equipment to look ‘sciencey’ and a little bit steampunk, Craig Neill (Senior Experimental Scientist in Ocean Carbon Observations at CSIRO) has just the thing.
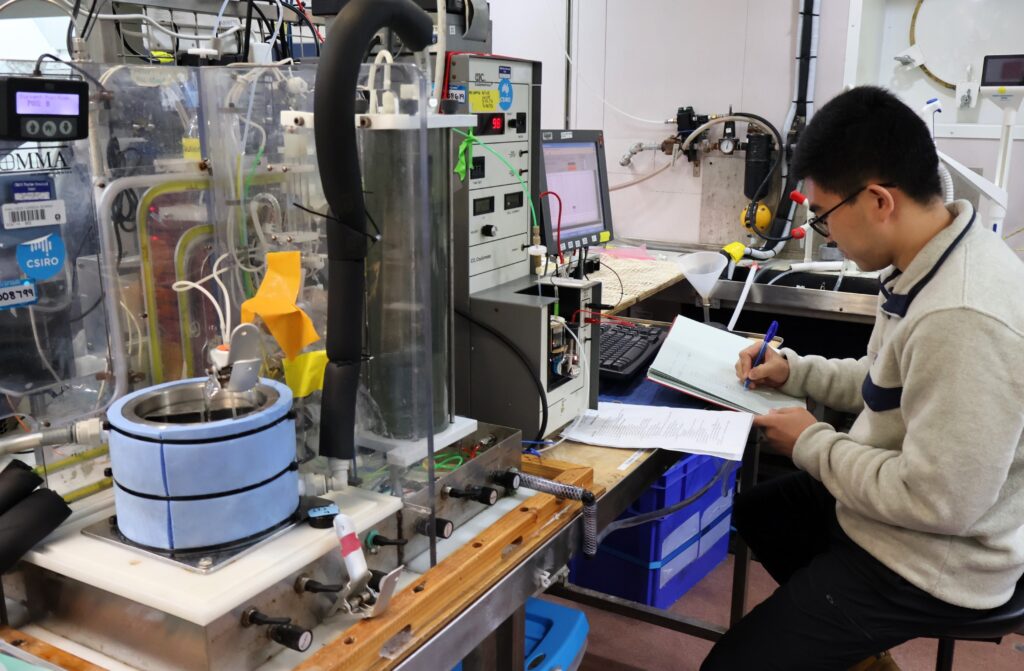
A world-leading expert in ocean carbon measurement, Craig has designed and built a Dissolved Inorganic Carbon analyser to replace the existing system that’s been in use for the last 30 years.
Moreover, he’s installed both old and new analysers on CSIRO research vessel (RV) Investigator, where they’ve been in constant use for the FOCUS voyage and one will remain in action for the upcoming MISO voyage in early 2024.
With the help of AAPP PhD student Xiang Yang, Craig is verifying the new system against the old one. With more than 500 water samples from the CTD rosette analysed by both of them on this voyage, he’s very pleased with the results.
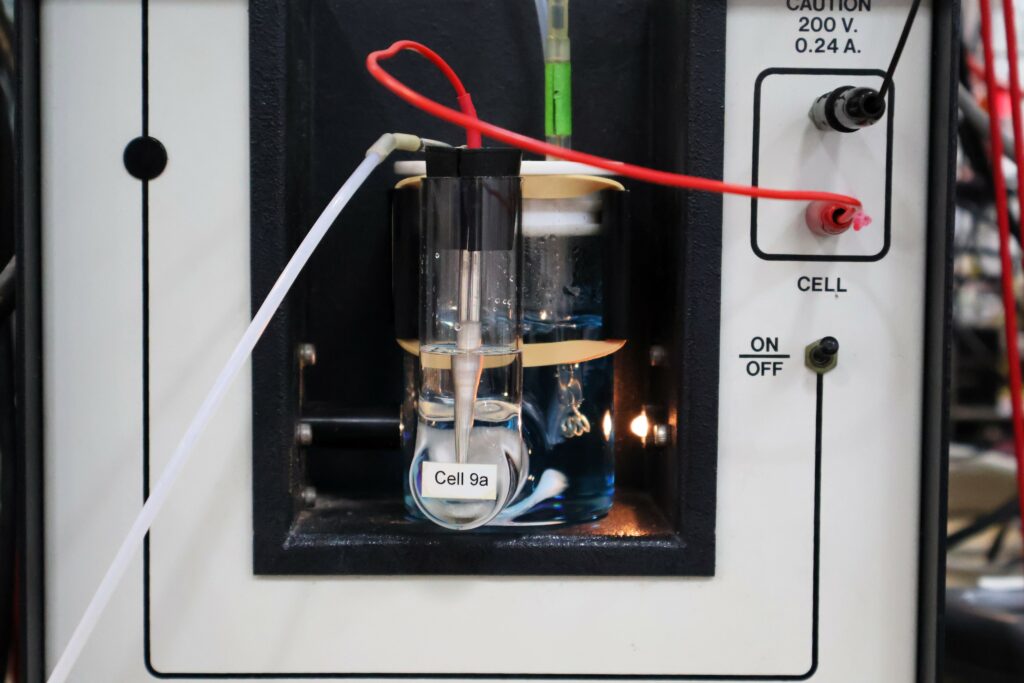
Craig actually trained with the inventor of the original system, the SOMMA (Single-Operator Multiparameter Metabolic Analyzer) coulometer-based system, in 1992.
A coulometric technique uses the amount of electric charge transferred in a chemical reaction as a proxy for measuring CO2 gas. The measure of the total amount of CO2 extracted from the sample is based on the electrical charge needed to maintain a constant pH in an electrochemical cell.
Craig says this is a tricky, sometimes temperamental process.
His new analyser measures dissolved carbon by collecting the CO2 extracted from the seawater sample and quantifying it with a laser spectrometer.
While currently all the samples for Dissolved Inorganic carbon analysis come from the CTD casts, the next goal is to adapt the analyser for integrating with another system that continuously measures surface ocean pCO2 in the underway sampling lab, to add the ability to automatically measure dissolved inorganic carbon every 30 minutes.
Why measure Dissolved Inorganic Carbon?
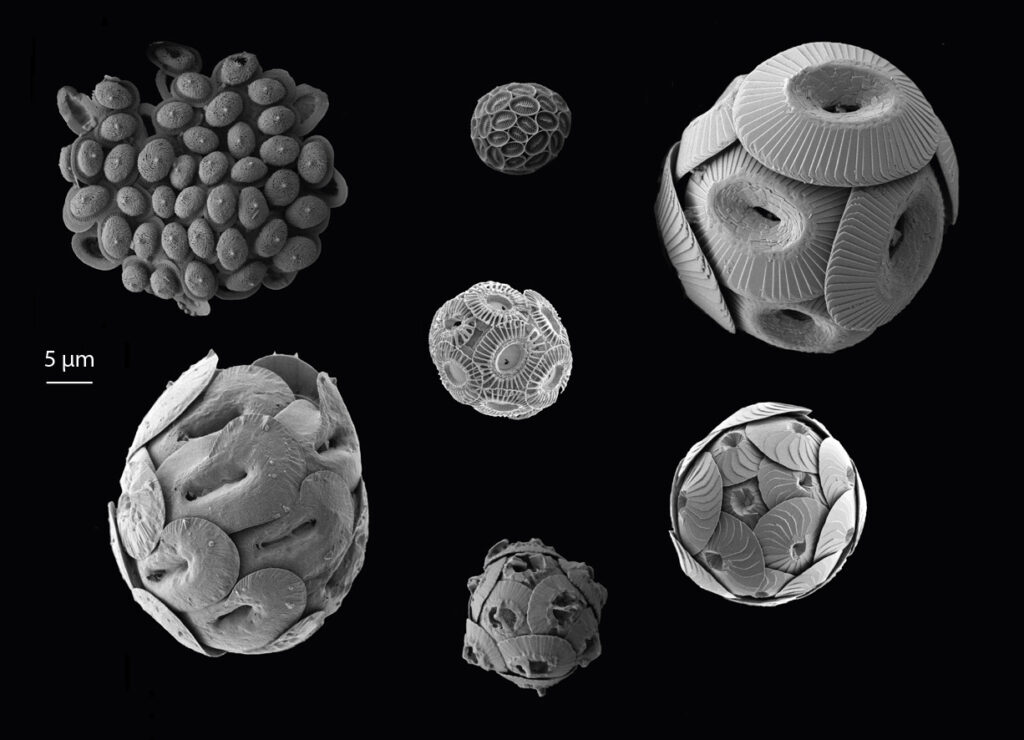
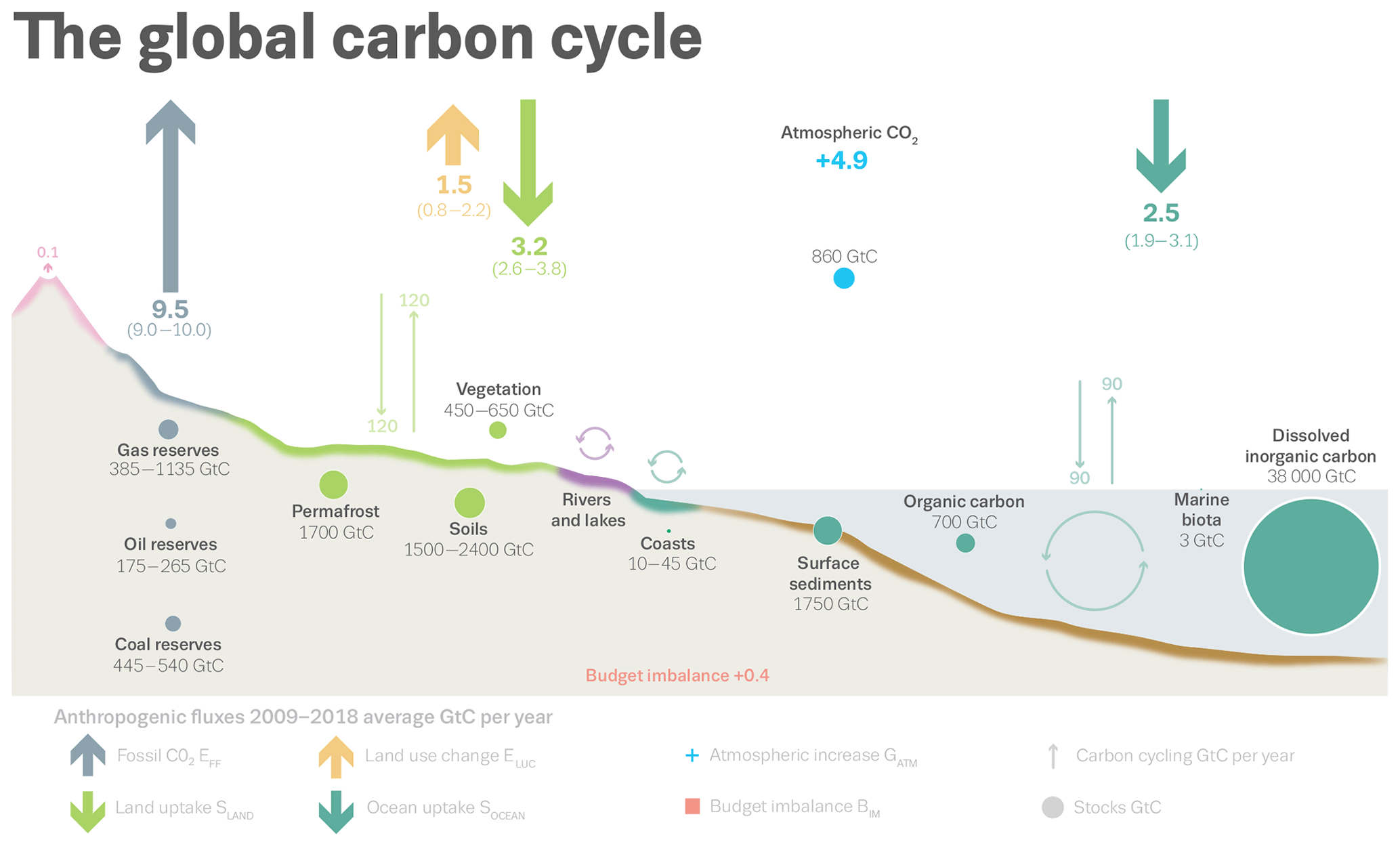
Quantifying Dissolved Inorganic Carbon (DIC) is important because the ocean is the largest reservoir of carbon on the planet, storing 38,000 Gt carbon (as DIC) with a residence time of ~100,000 years (by comparison, the atmospheric carbon stock is ~860 Gt).
DIC in seawater consists of carbon in three forms: carbon dioxide (CO2), bicarbonate (HCO3), and carbonate (CO3). The relative abundance of these three carbon compounds is controlled by the pH of the water.
Because of the continuous exchange between the surface of the ocean and the atmosphere, and the way CO2 reacts with seawater, the ocean has absorbed about 25% of human-caused CO2 emissions, with some 40% of this stored in the Southern Ocean. This climate service comes at a cost: the change in seawater chemistry leads to an increase in the oceanic CO2 and a coincident decrease in pH (more acidic) and carbonate saturation state, a chemical threshold that determines the energetic demand to marine calcifiers from ocean acidification – organisms building shells of skeletons from calcium carbonate.
This work is linked to ongoing efforts by the CSIRO Ocean Carbon Observations Team and the AAPP Biogeochemistry Project to quantify the uptake and storage of CO2 in the Southern Ocean. The team uses observations from voyages like the FOCUS campaign, and also from autonomous platforms.
Recently a compilation of nearly a decade of CO2 observations from the Southern Ocean Time Series observatory (SOTS) in the Sub-Antarctic Zone to the north of the FOCUS study region revealed changes in ocean chemistry resulting from anthropogenic CO2 uptake.
This research is supported by a grant of sea time on RV Investigator from the CSIRO Marine National Facility which is supported by the Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS).